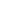Transdermal and Combined Oral
Contraceptive (COC) PK profiles
In general, overall exposure for NGMN and EE (AUC and Css) was higher in subjects treated with norelgestromin and Ethinyl Estradiol transdermal system for both Cycle 1 and Cycle 2, compared to that for the oral contraceptive. Cmax values were higher in subjects administered the oral contraceptive. Under steady state conditions, AUC0-168 and Css for EE were approximately 55% and 60% higher, respectively, for norelgestromin and Ethinyl Estradiol transdermal system, and the Cmax was about 35% higher for the oral contraceptive, respectively.
Inter-subject variability (%CV) for PK parameters following delivery from NGMN and EE transdermal system was higher relative to the variability determined from the oral contraceptive. The mean PK profiles are different between the two products and caution should be exercised when making a direct comparison of these PK parameters.
Ethinyl Estradiol Exposure
Higher estrogen exposure may increase the risk of adverse reactions, including venous thromboembolism (VTE). The Area Under the Curve (AUC) for Ethinyl Estradiol (EE) is approximately 60% higher in women using XULANE compared to oral contraceptives containing EE 35 mcg. In contrast, the peak concentration (Cmax) for EE is approximately 25% lower in women using norelgestromin and Ethinyl Estradiol transdermal system [See Section 12.3 (Clinical Pharmacology) in the XULANE PI for more information].
The figures below present mean PK profiles for NGMN and EE, respectively,
following once-daily administration of an oral contraceptive (containing NGM
250 mcg /EE 35 mcg) compared to the 7-day norelgestromin and Ethinyl
Estradiol transdermal system (containing NGMN 4.86 mg/EE 0.53 mg) during
Cycle 2 in 32 healthy female volunteers.
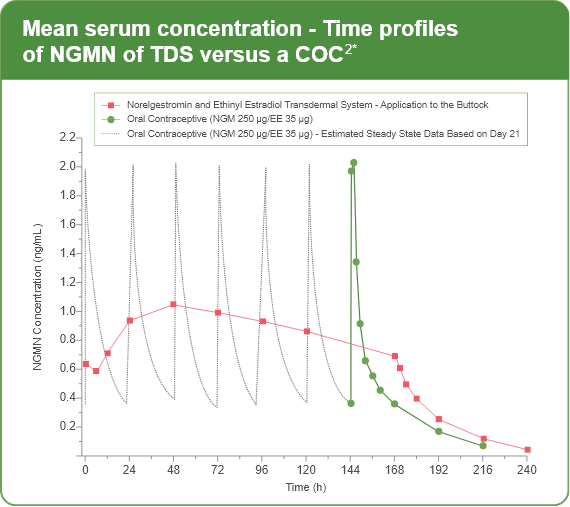
*Following once-daily administration of an oral contraceptive for two cycles or application of NGM for two cycles to the buttock in healthy female volunteers.
[Oral contraceptive: Cycle 2, Days 15 to 21, XULANE: Cycle 2, Week 3]
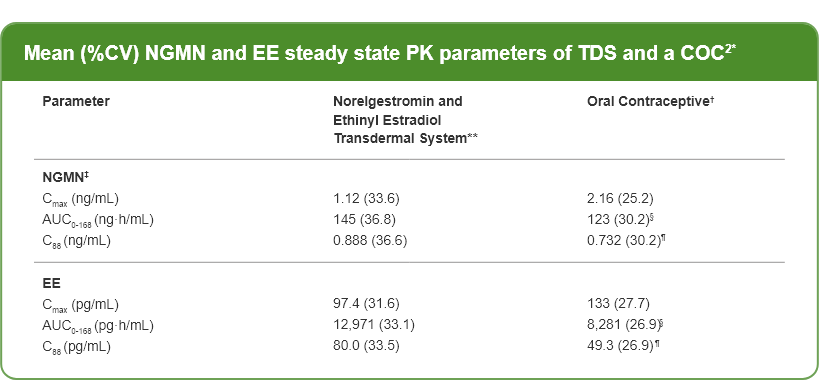
* Mean (%CV) NGMN and EE steady state pharmacokinetic parameters following application of norelgestromin and
Ethinyl Estradiol transdermal system and once-daily administration of an oral contraceptive (containing NGM 250
mcg /EE 35 mcg) in healthy female volunteers
** Cycle 2, Week 3
† Cycle 2, Day 21
‡ NGM is rapidly metabolized to NGMN following oral administration
§ Average weekly exposure, calculated as AUC24 x 7
¶ Cavg
VTE: Venous Thromboembolism; COCs: Combined Oral Contraceptives; AUC: Area Under the Curve; EE: Ethinyl Estradiol; NGMN: Norelgestromin. TDS: Transdermal System